

Drug Discovery and Development Flow Chart




Questions
Where are you in the overall process?
What studies need to be completed in each stage?
Which studies need to be conducted under GLP Regulations and ICH guidelines?
Should any of the required studies be outsourced? If so, to whom?
How should the completed studies be documented?
What should be included in the IND? In the NDA?
Which preclinical studies are necessary for product extensions? Clinical studies?
Estimated Accumulative Cost - Time Curve for Drug Discovery and Development
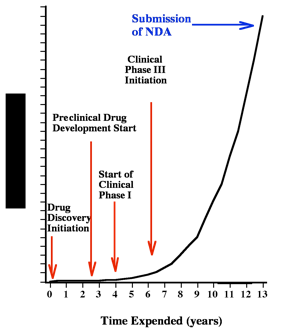
Where are you on the cost - time curve?
How can the optimal discovery lead be selected for further development?
Does your drug development logic plan list all the studies necessary for an IND submission? For a NDA submission?
Are in vitro and animal studies predictive of safety in humans? Efficacy? Delivery? Distribution?
Were the early studies well designed and data productive so that expensive clinical trials do not have to be repeated?












